All the below tweets are in English.
標記につき取り急ぎ以下貼っておきます。
Spreads Quickly In Air: Vietnam On Hybrid Of Variants Found In India, UK
Read more: https://t.co/1hsseLZGUx#COVID19 pic.twitter.com/VsyThFtjkZ
— NDTV (@ndtv) May 30, 2021
A second highly infections #COVID19 variant that’s overwhelmed India and is spreading in the UK has been found in #Melbourne.
The Delta strain is more transmissible than its Kappa cousin – blamed for our lockdown. @msanto92 #9News pic.twitter.com/cNGPcnRovw
— 9News Melbourne (@9NewsMelb) June 4, 2021
Authorities in #Vietnam have detected a new #coronavirus variant that is a combination of the #Indian and #UK #COVID-19 variants and spreads quickly by air, the health minister of Vietnam said on Saturday.#india #news #covidnews #COVID19 #UPnews https://t.co/YB5P0B1q3O
— तरुणमित्र (हिंदी दैनिक ) (@tarunmitragroup) June 4, 2021
Under the new scheme, first varrient identified in the UK, is labelled “alpha”. The 2nd one from South Africa is “beta”, the 3rd one from Brazil is “gamma” and the 4th one detected in India is “delta”.#COVID19 #COVID19PK
https://t.co/vQONPBkXGK— Muzzamil Hussain مزمل حسین (@1sudo) June 4, 2021
#COVID19 variant first found in the UK will be labelled ‘Alpha’ and the one detected in India as ‘Delta’, according to WHO's new nomenclature
Read more in today's #HTCovidDispatch: an informed pandemic-related newsletter with updates and opinions https://t.co/zFdS5POLlk pic.twitter.com/FS7wei4Tfw
— Hindustan Times (@htTweets) June 1, 2021
https://twitter.com/katyjon/status/1399694623015161860
https://twitter.com/MissManc/status/1399833131469971458
From now on the WHO will use Greek letters to refer to variants of #COVID19.
Current variants of concern (VOC) are:
Alpha – first identified in UK
Beta – first identified in South Africa
Gamma – first identified in Brazil
Delta – first identified in India https://t.co/loTb61rv9e— Junayd (@mjunayd) May 31, 2021
Vaccilating on Vaccines@NepaliTimes Editorial: 'Nepal’s vaccine diplomacy has failed, but India, UK, EU, US, WHO have also let us down as we are battered by the #Covid19 second wave."https://t.co/24I2vCsv8D
— Kunda Dixit (@kundadixit) May 30, 2021
WHO: #Covid19 variants to be given Greek alphabet names to avoid stigma. https://t.co/XSNvwhiDiG
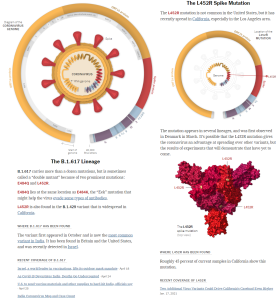
4 present variants of concern—UK/Kent (B.1.1.7), South Africa (B.1.351), Brazil (P.1) & India (B.1.617.2)—will now be given the letters Alpha, Beta, Gamma & Delta respectively.
— 🚶🏻Curtis S. Chin (@CurtisSChin) June 1, 2021
#COVID19 variant identified in #India may increase risk of hospitalization, UK officials say https://t.co/pXq230DK7K
— Saudi Gazette (@Saudi_Gazette) June 4, 2021
Panel warns #India’s ‘delta’ #COVID19 variant highly infectious. Global response needed to tackle virus that is 50% more transmissible than the one in UK https://t.co/leXkS2vqGT
— Gulf News (@gulf_news) June 4, 2021
Prior infections and partial vaccinations are insufficient impediments to its spread, as seen in Delhi, say researchers. #FMTNews #India #Covid19 https://t.co/r0QnklFOrk
— Free Malaysia Today (@fmtoday) June 4, 2021
As #India 🇮🇳 battles the second wave of #COVID19 , a critical shortage of oxygen for patients has posed a major challenge.
UNICEF continues its emergency support by providing additional lifesaving supplies. Read more: https://t.co/kxjs1eGRql pic.twitter.com/RuYOZyuEoU
— UNICEF UK Media (@Unicefuk_media) June 3, 2021
UK reports no new #Covid19 deaths for the first time since March 2020
LIve: https://t.co/lZoU0GUiNg#Coronavirus #covid19 pic.twitter.com/EHCJlq0UJN— IndiaToday (@IndiaToday) June 2, 2021
Excellent. We shouldn't be classifying diseases based on country of origin. Having this terminology for #covid19 variants is important (even as I wish they'd come earlier):
Alpha: B.1.1.7 (UK)
Beta: B.1.351 (South Africa)
Gamma: P.1 (Brazil)
Delta: B.1.617.2 (India) https://t.co/q6kzpZJKBM— Leana Wen, M.D. (@DrLeanaWen) June 1, 2021
.@IndianExpress reports India may soon test feasibility of mixing two different vaccines. Early data from a similar study in UK showed adverse reactions were short lived, with no other safety concerns. Full results coming in June. https://t.co/uggzxwWcfD #Covid19 #vaccines
— Soutik Biswas (@soutikBBC) May 31, 2021
"Covid has no credible natural ancestor": A new explosive UK study claims that #coronavirus was actually created in a lab in Wuhan#ITVideo #China #Wuhan #WuhanLab #Coronavirus #Covid19 #UKStudy pic.twitter.com/2Tg3P6virF
— IndiaToday (@IndiaToday) May 31, 2021
Vietnam plans to test all 9 million people in its largest city for the coronavirus and imposed more restrictions Monday to deal with a growing #Covid19 outbreak.https://t.co/flfO34FKY3
— Hindustan Times (@htTweets) May 31, 2021
#Vietnam has discovered a new coronavirus variant that's a hybrid of strains first found in #India and the #UK.#COVID19 https://t.co/YNcEBU1r0K
— Outlook Magazine (@Outlookindia) May 30, 2021
Vietnam detects hybrid of Indian and UK Covid-19 variant #Vietnam #COVID19 #Indiahttps://t.co/rRUedZ36nb pic.twitter.com/HtZG2dIlSB
— 92 News HD Plus (@92newschannel) May 29, 2021
Vietnam has discovered a new #Covid19 variant that’s a hybrid of #coronavirus strains first found in India and the U.K.https://t.co/va39hzVpLe
— The New Indian Express (@NewIndianXpress) May 29, 2021
Vietnam detected a highly transmissible new variant of the #coronavirus that has helped fuel a recent wave of infections in the country.
Genetic sequencing indicates that the new variant is a mix of the strains first detected in the UK & India.https://t.co/yrK2cPlpEX #COVID19
— MicrobesInfect (@MicrobesInfect) May 29, 2021
https://twitter.com/DrEricDing/status/1398631148927004675
Vietnam detects hybrid of Indian, UK #COVID19 variants https://t.co/aU6wYhz0YR
— Business Today (@BT_India) May 29, 2021
"After running gene sequencing on newly detected patients, we have discovered a new variant that is a mix of India and UK ones," Nguyen Thanh Long was quoted as saying.#COVID19
— Deccan Herald (@DeccanHerald) May 29, 2021
Vietnam detects hybrid of the COVID-19 variant first detected in the UK and the variant first detected in India #COVID19 https://t.co/IcPb9Os0qd
— Sean Previl (@SeanPrevil) May 29, 2021
"We found 94% of the patients with #COVID19 can be treated with home care."@Deloitte CEO @PunitRenjen discusses his company's work with the #Haryana govt to set up a virtual health care system & reduce the "crush" on #India's hospitals.
His mother & employees included ❤️ pic.twitter.com/NmtnhvW4vt
— Julia Chatterley (@jchatterleyCNN) May 25, 2021
https://twitter.com/trtworld/status/1398967555692314626
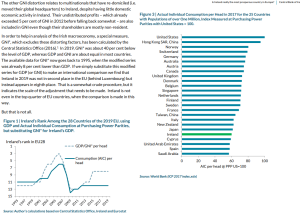
USA
2019 : 3.67% | 2020 : 8.31%Germany
2019 : 3.14% | 2020 : 4.31%UK
2019 : 3.74% | 2020 : 4.34%Sweden
2019 : 6.83% | 2020 : 8.45%World
2019 : 5.37% | 2020 : 6.47%India
2019 : 5.27% | 2020 : 7.10%(Source : World Bank)#CongressToolkitExposed pic.twitter.com/DYPmJZM4g6
— Abhijeet Srivastava 🇮🇳 (@abhijeetrai00) June 2, 2021
https://twitter.com/BiIndia/status/1400520157181612036
https://twitter.com/BiIndia/status/1399734985586659332
#COVID19 | The research paper was published on the backdrop of the UK's decision to reduce the gap between two doses of vaccine to address the concerns about the spread of the B.1.617 variant, first detected in India.https://t.co/VnIBRZFMYp
— Hindustan Times (@htTweets) June 4, 2021
The UK officials working on the review into COVID-19 status certificates believe there is no chance the law will be changed to mandate their use within the UK, the report added.#Covid19 https://t.co/YrX4RaEJRc
— IndiaToday (@IndiaToday) May 31, 2021
Delta Variant of Covid-19 Now Dominant in UK, May Have Higher Hospitalisation Risk: Health Officialshttps://t.co/7xIV6C5uAk#COVID19 #coronavariant #UK #DeltaVariant
— Yahoo India (@YahooIndia) June 5, 2021
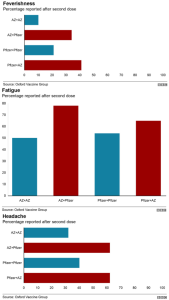
The Delta variant of #COVID19, or the B1.617.2 highly transmissible variant of concern (VOC) first identified in India, has now become the dominant VOC in the #UK as infections rose by 5,472 in a week to hit a total of 12,431 https://t.co/2DbcjKfuMJ
— National Herald (@NH_India) June 4, 2021
The Delta variant of concern first identified in India is now dominant in Britain and might have an increased risk of hospitalisation compared to the Alpha variant, #UK #Covid19 #CovidVarianthttps://t.co/zTRsjBI97w
— The Peninsula Qatar (@PeninsulaQatar) June 4, 2021
COVID-19 #LIVE Updates | UK Approves Pfizer/BioNTech COVID Shot For Kids In 12-15 Age Group@pfizer #COVID19 #CoronavirusPandemic #Vaccine https://t.co/pvxuzpzMM3
— NewsMobile (@NewsMobileIndia) June 4, 2021
via @NYTimes Delta variant leaves UK cautious despite announced June 21 opening up. US on the other hand has dropped covid19 restraints. Europe is even more cautious. Unlikely if India will see barred entry to these nations lifted soon. https://t.co/UwOE8axlWW
— K. C. Singh (@ambkcsingh) June 5, 2021
https://twitter.com/FISI_UK/status/1400025769573761024
The #JohnsonVariant of #COVID19 is spreading in England's #schools and colleges, @PHE_uk data shows@BorisJohnson @Conservatives #coronavirus #Sage @IndependentSage https://t.co/AFm2eTwqGH
— Noise/Clinic (@NoiseClinic1) June 4, 2021
Concerns over #IndianVariant risk with more #NHS Hospital admissions…#COVID19 #Covid19UKhttps://t.co/6vuU0SiB3x
— Oinfo UK (@oinfo_uk) June 4, 2021
Despite a fairly high vaccination rate,the UK has started experiencing a new surge in #COVID19 cases during last 7 days. There has been a 24% jump.
Another indicator of the fact that India should be cautious in unlock moves and do not lower the guards. pic.twitter.com/ZXvOaFxUys— Pranay Upadhyaya (@JournoPranay) May 29, 2021
UK reports 5,765 new cases of #COVID19 on Saturday and 13 more deaths within 28 days of a positive test pic.twitter.com/Gbgw4NOuL0
— DD India (@DDIndialive) June 5, 2021
Scientists in UK urge caution over lockdown end amid COVID third wave fears#COVID19 #unitedkingdom https://t.co/Ocl1BNEUEe
— India TV (@indiatvnews) June 1, 2021
The #UK is in the early stages of a third wave of #COVID19, a scientist advising the UK government said, media reports said on Monday. pic.twitter.com/zmqGtCcnNU
— IANS Tweets (@ians_india) May 31, 2021
#London’s busy #HeathrowAirport opened a dedicated new terminal on Tuesday for arrivals from countries designated as “red list”, such as #India, for a higher risk of #COVID19 transmissionhttps://t.co/tpvMgCR0xn
— FinancialXpress (@FinancialXpress) June 1, 2021
Currently, there are 43 countries on the UK government's red list to cover regions linked with high-risk Covid-19 variants #coronavirus #COVID19 https://t.co/qDyYVzHIu0
— Khaleej Times (@khaleejtimes) June 1, 2021
Currently, there are 43 countries on the UK government’s red list to cover regions linked with high-risk COVID-19 variants such as #India, Brazil and South Africa.#RedList #COVID19 #travel https://t.co/woQzq3X0Wy
— Travel Turtle (@travelturtlemag) June 1, 2021
#BorisJohnson dithered allowing a minimum 20,000 from India before a half-arsed ‘ban’, which STILL allows flights daily and now his #IndianVariant is rampant throughout the UK ….
holidays f****d again thanks to #BorisTheClown #COVID19 https://t.co/jy1tzpYnlF— geoffh (@geoffh33) June 3, 2021