All the below links and excerpts are in English.
Sarilumab (Kevzara®) に係るリンクと当方作成の抜粋を取り急ぎ以下のとおり貼っておきます。
cf.
Science and Technology 科学技術 Vol.14 / #Coronavirus #コロナウイルス Vol.15(pharmaceutical products 医薬品 Vol.6:drugs 治療薬) Tocilizumab
Science and Technology 科学技術 Vol.15 / #Coronavirus #コロナウイルス Vol.16(pharmaceutical products 医薬品 Vol.7:drugs 治療薬)
Clinicians encouraged to consider tocilizumab or sarilumab in treatment of hospitalised COVID-19 patients (07/01/2021) | @PJOnline_News,@robinson_julia
… The new advice, which is due to be published on 8 January 2020, follows results released from the Randomised, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired pneumonia (REMAP-CAP), which suggested that tocilizumab and sarilumab, which are both IL-6 receptor antagonists, led to a 24% reduced risk of mortality, when administered to patients within 24 hours of entering intensive care. …
At the time of full analysis, 353 patients had been assigned to tocilizumab, 48 to sarilumab and 402 to standard care. The majority of patients were also treated with corticosteroids, such as dexamethasone, and were receiving respiratory support.
The trial data yielded an odds ratio of 1.64 for a better outcome with tocilizumab, and 1.76 for sarilumab, compared to no immune modulation, with a high degree of statistical certainty.
…Anthony Gordon…
Gino Martini, chief scientist at the Royal Pharmaceutical Society…
Tocilizumab and sarilumab have already been added to the government’s export restriction list, which bans companies from buying medicines meant for UK patients and selling them on for a higher price in another country. This will protect supply for UK patients by enforcing regulatory action on those who flout the restrictions.
Roche’s Actemra, Regeneron’s Kevzara win U.K.’s favor in COVID-19 after study shows 24% drop in death risk (01/08/2021) | @FiercePharma,@arleneweintraub
The question of whether seriously ill COVID-19 patients can benefit from anti-inflammatories like Roche’s Actemra and Sanofi and Regeneron’s Kevzara has dogged practitioners in the United States thanks to conflicting clinical trial results.
The United Kingdom, on the other hand, has reached a definitive answer on the two drugs, both of which are IL-6 inhibitors: They significantly reduce the risk of death in COVID-19 patients needing intensive care, and they should be used to ease the pressure hospitals are now facing as the coronavirus pandemic continues to intensify, the country’s National Institute for Health Research (NIHR) said Thursday.
The recommendation came after data from an NIHR-sponsored study showed that Actemra and Kevzara can cut hospital stays for COVID-19 patients admitted to intensive care by 10 days and can lower the risk of death by 24% in patients who receive either drug within a day of admission. That finding prompted the U.K. government to recommend to the National Health Service (NHS) that IL-6 inhibitors be rolled out for the treatment of COVID-19. …
UK Covid patients to receive new drugs tocilizumab and sarilumab that reduce death risk by 24% (w Video; 01/08/2021) | @EveningStandard,@HattieBrewis
… NHS patients admitted to the country’s intensive care units will be able to receive tocilizumab and sarilumab, which have also been found to reduce the time coronavirus sufferers need to spend in hospital by up to 10 days. …
It comes after results from the Government-funded REMAP-CAP clinical trial showed that both drugs reduced the risk of mortality by 8.5 per cent when administered to patients within a day of entering intensive care alongside a corticosteroid, such as dexamethasone.
Professor Anthony Gordon, chair in anaesthesia and critical care at Imperial College London and a consultant in intensive care medicine at Imperial College Healthcare NHS Trust…
Hospital mortality was 27.3 per cent among patients receiving tocilizumab or sarilumab, compared with 35.8 per cent of patients in the control group who did not receive the drugs, the researchers said.
Treatment with tocilizumab or sarilumab is thought to cost somewhere between £750 and £1,000 and is administered intravenously either as a one or two dose regime.
Professor Gordon added: “For every 12 patients in intensive care you treat with these drugs, based on the evidence we saw, you would expect to save one life.” …
Tocilizumab and sarilumab have been added to the Government’s export restriction list, the Department of Health and Social Care (DHSC) said.
This will protect supply for UK patients by enforcing regulatory action on those who flout the restrictions, it added.
Commenting on the findings, Peter Horby, professor of Emerging Infectious Diseases, the University of Oxford’s Centre for Tropical Medicine and Global Health…
“Importantly, the findings of a benefit are in addition to corticosteroids, which were taken by 93 per cent of the participants.
“This means we now have two drugs, which combined have a greater effect.”
KEVZARA HELPS INHIBIT THE EFFECTS OF CHRONICALLY ELEVATED IL-6: KEVZARA targets and binds with high affinity to soluble and membrane-bound IL-6 receptors (sIL-6R and mIL-6R), thereby inhibiting IL-6 signaling | @sanofi & @Regeneron

UK approves anti-inflammatory drugs to treat sickest covid-19 patients after strong results in clinical trial (01/08/2021) | @washingtonpost,@Carolynyjohnson,@bbguari
…Jonathan Parr…
David E. Leaf, a Harvard Medical School assistant professor and Brigham and Women’s Hospital physician, said the data were game-changing: “For ICU patients, I think it is a slam dunk that they should be given tocilizumab if it can be given early on.”…
“We’re currently live in 290 sites around the world, and so there was no way to quietly whisper to 290 sites: ‘Stop randomizing to control in the immune modulation arm, but don’t tell anyone,’ ” said Derek Angus, chair of critical care medicine at the University of Pittsburgh Medical Center and one of the investigators of the trial. …
The list price of the dose of Kevzara used in the trial is about $3,600. The maximum dose used in the trial of Actemra currently carries a list price of $4,600, but the dose varied by patients’ body weight and could be given either once or twice. Bob Purcell, a spokesman for Genentech, a member of the Roche Group, said that the drug was not approved for use in covid-19 and the pricing might differ if it were approved to treat the illness. …
About 36 percent of patients died in the hospital who received standard care, while 28 percent died on tocilizumab and 22 percent died when given sarilumab. Patients treated with the drugs spent about a week less in the ICU, on average. …
UK Approves Arthritis Drugs for Critically Ill COVID-19 Patients (01/11/2021) | @TheScientistLLC,@AsherGJones
… The study, which has not yet been peer-reviewed, evaluated around 800 patients in intensive care with severe COVID-19. Around half received the standard of care while 353 received tocilizumab and 48 received sarilumab. The researchers found that 35.8 percent of those given standard care died, compared with 28 percent who received tocilizumab and 22.2 percent who were given sarilumab. …
The immunosuppressive drugs tocilizumab and sarilumab, which are primarily used to treat arthritis, block signaling of the cytokine IL-6, a key regulator of immune dysregulation and inflammation in severe COVID-19 cases. …
…Krutika Kuppalli, an infectious disease physician at the Medical University of South Carolina… @nytimes
…Jonathan Parr, an infectious diseases physician at the University of North Carolina at Chapel Hill… @washingtonpost
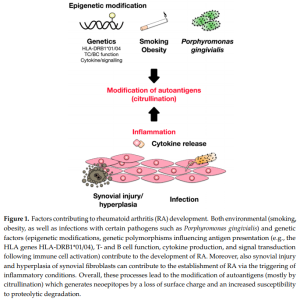
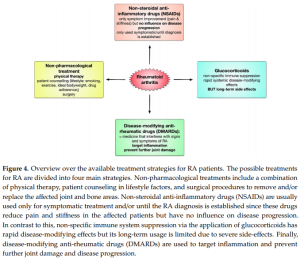
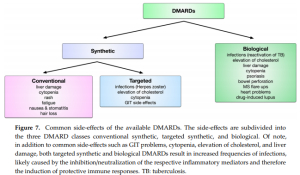
Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis (04/03/2021) | National Center for Biotechnology Information, U.S. National Library of Medicine
Kevzara (Sarilumab injection, for subcutaneous use): side effects (05/18/2018) | @RxList
Kevzara (sarilumab) injection is an interleukin-6 (IL-6) receptor antagonist indicated for treatment of adult patients with moderately to severely active rheumatoid arthritis (RA) who have had an inadequate response or intolerance to one or more disease-modifying antirheumatic drugs (DMARDs). Common side effects of Kevzara include:
・low white blood cell count (neutropenia),
・increased ALT,
・injection site redness,
・upper respiratory infections,
・nasal congestion,
・runny nose,
・sore throat,
・urinary tract infections, and
・low platelet counts (thrombocytopenia). …
Sarilumab (Kevzara®) Drug Information Sheet | @jhrheumatology

Kevzara Side Effects (09/13/2020) | @Drugscom
Side effects requiring immediate medical attention
… Check with your doctor immediately if any of the following side effects occur while taking sarilumab:
More common
・Bloody, black, or tarry stools
・chills
・cough
・fever
・lower back or side pain
・painful or difficult urination
・pale skin
・sore throat
・ulcers, sores, or white spots in the mouth
・unusual bleeding or bruising
・unusual tiredness or weakness
Less common
・bladder pain
・bloody or cloudy urine
・body ache or pain
・difficulty breathing
・ear congestion
・frequent urge to urinate
・headache
・loss of voice
・nasal congestion
・painful cold sores or blisters on the lips
・runny nose
・sneezing
Rare
・difficulty swallowing
・dizziness
・fast heartbeat
・heartburn
・hives, itching, skin rash
・indigestion
・nausea
・puffiness or swelling of the eyelids or around the eyes, face, lips or tongue
・severe stomach pain, cramping, or burning
・tightness in the chest
・vomiting of material that looks like coffee grounds, severe and continuing
Side effects not requiring immediate medical attention
More common
・bleeding, blistering, burning, coldness, discoloration of skin, feeling of pressure, infection, inflammation, itching, lumps, numbness, pain, rash, redness, scarring, soreness, stinging, swelling, tenderness, tingling, ulceration, or warmth at the injection site
For Healthcare Professionals
Dermatologic; Hematologic; Hypersensitivity; Immunologic; Local; Metabolic; Genitourinary; Hepatic; Respiratory; General; Gastrointestinal
Kevzara (Sarilumab), a New IL-6 Receptor Antagonist, Approved for Active Rheumatoid Arthritis (03/2018) | American Health & Drug Benefits @TheLynxGroup
Mechanism of Action
Sarilumab is a human recombinant monoclonal antibody that binds to IL-6 receptors that inhibit IL-6?mediated signaling. The IL-6 cytokine plays a role in the body’s inflammatory process and response. The production of IL-6 by synovial and endothelial cells can lead to local production of IL-6 in the joints affected by RA and other inflammatory processes. Elevated levels of IL-6 have been correlated with disease activity and joint damage in patients with RA.
Dosing and Administration
The recommended dosage of sarilu-mab is 200 mg once every 2 weeks, administered as a subcutaneous injection; it is available as a 150-mg/1.14-mL or 200-mg/1.14-mL solution in a single-dose, prefilled syringe, and can be used as monotherapy or in combination with methotrexate or other conventional DMARDs. …
Adverse Reactions
The most common (incidence ≧3%) adverse reactions associated with saril-umab therapy are neutropenia (7%), increased alanine aminotransferase levels (5%), injection-site erythema (5%), upper respiratory infections (4%), and urinary tract infections (3%).
Drug Interactions
Cytokines and cytokine modulators can influence the activity of specific cytochrome (CY) P450 enzymes (including CYP3A4) and thus may alter the metabolism of drugs that are substrates of these enzymes. Use caution when sarilumab is co-administered with CYP3A4 substrate drugs for which decreased effectiveness is undesirable (eg, oral contraceptives, statins).
Use in Specific Populations
Nursing women may need to discontinue sarilumab or discontinue nursing.
No differences in safety or efficacy of sarilumab were seen between older (aged ≧65 years) and younger patients.
Warnings and Precautions
The prescribing information for saril-umab includes a boxed warning stating that sarilumab is associated with an increased risk for serious and potentially fatal infections, including bacterial, viral, invasive fungal, and other opportunistic infections. Patients should be monitored closely for infection while receiving sarilumab.
Sarilumab has been associated with reduced ANC, including neutropenia; a reduction in platelet counts; transaminase elevations; and lipid abnormalities.
The risk for gastrointestinal perforation may be increased when sarilumab is used concomitantly with NSAIDs or with corticosteroids.
Treatment with immunosuppressant drugs, including sarilumab, may increase the risk for malignancies.
Sarilumab should be discontinued immediately if anaphylaxis or a hypersensitivity reaction occurs.
Sarilumab should not be used with live vaccines.
* Kevzara (sarilumab) injection [prescribing information]. Tarrytown, NY: Regeneron Pharmaceuticals; Bridgewater, NJ: sanofi-aventis U.S.; May 2017.
Repurposed Drugs to Fight SARS-CoV-2, Tocilizumab and Sarilumab – Part 4 (04/15/2021) | @_Monocl
Tocilizumab and sarilumab reduce COVID-19 patient mortality by 8.5 percent, data shows (07/01/2021) | @PharmaReview
Kevzara (sarilumab) | @mnt
Sarilumab (Subcutaneous Route) | @MayoClinic
Efficacy and safety of sarilumab in patients with active rheumatoid arthritis (01/11/2018) | National Center for Biotechnology Information, U.S. National Library of Medicine
Sarilumab | @sciencedirect
