All the below links are in English. Excerpts are on our own.
ご参考まで取り急ぎ以下貼っておきます。
cf.
World Vol.215 (coronavirus – Omicron variant)
UK Vol.189 (coronavirus – Omicron variant)
Pfizer Shares In Vitro Efficacy of Novel COVID-19 Oral Treatment Against Omicron Variant (01/18/2022) | @Pfizer
…the in vitro efficacy of nirmatrelvir, the active main protease (Mpro) inhibitor of PAXLOVID (nirmatrelvir [PF-07321332] tablets and ritonavir tablets), is maintained against the SARS-CoV-2 variant Omicron. …PAXLOVID has the potential to maintain plasma concentrations many-fold times higher than the amount required to prevent Omicron from replicating in cells. …
…PAXLOVID reduced risk of hospitalization or death by nearly 90% compared to placebo for high-risk patients when treated within five days of symptom onset…
Current VoCs * can be resistant to treatments that work by binding to the spike protein found on the surface of the SARS-CoV-2 virus. PAXLOVID, however, works intracellularly by binding to the highly conserved Mpro of the SARS-CoV-2 virus. Previous data have also indicated that PAXLOVID maintains in vitro efficacy against earlier and current VoCs, including Alpha, Beta, Gamma, Delta, Lambda, and Mu.
PAXLOVID is currently authorized for conditional or emergency use in several countries across the globe. Pfizer has submitted applications for regulatory approval or authorization to multiple regulatory agencies and anticipates further regulatory decisions to follow.
* VoCs = variants of concern
‘There are very limited treatments’: COVID outpatient treatments evaporate with omicron (w Video; 01/17/2022) | @WLWT
… The oral COVID-19 treatments from Pfizer and Merck are expected to be effective in treating outpatients, but manufacturing hasn’t ramped up to the point that the pill-form treatments are readily available. …
‘Omicron patients under 60 with no comorbidities can start symptomatic treatment with paracetamol’ (01/18/2022) | @IndianExpress
…a 30 per cent hospitalisation risk reduction with the use of molnupiravir. If someone above the age of 50 has a consistent fever for two days alongside two or three comorbidities, the doctor might prescribe them these antivirals. …
…there is no need to worry as these pills are not prescribed to children below the age of 18, or to patients who require hospitalization, or to pregnant women. “The pill must be administered within 72 hours of the onset of symptoms for patients in the high-risk population group, namely, hypertensives, diabetics, people on immunosuppressed medication, senior citizens, people with other ailments etc.”…
WHO adds new drugs to COVID treatments amid Omicron surge (14/01/2022) | @AJEnglish
… The drug baricitinib, which is also used to treat rheumatoid arthritis, is “strongly recommended” for patients with severe or critical COVID-19, in combination with corticosteroids…
The panel also gave a “conditional recommendation” for sotrovimab, an experimental monoclonal antibody treatment, for those with non-severe COVID-19 but at the very highest risk of hospital admission. Monoclonal antibodies are lab-created compounds that mimic the body’s natural defence mechanism. …
The “guidance adds to previous recommendations for the use of interleukin-6 receptor blockers and systemic corticosteroids for patients with severe or critical covid-19; conditional recommendations for the use of casirivimab-imdevimab (another monoclonal antibody treatment) in selected patients; and against the use of convalescent plasma, ivermectin and hydroxychloroquine in patients with covid-19 regardless of disease severity,” the WHO said in a statement. …
Baricitinib is produced by United States pharmaceutical giant Eli Lilly, and while generic versions are available in India and Bangladesh, patents are in force in many other countries including Brazil and Indonesia. …
“The possibilities for providing high-level intensive care are limited, so saving more lives of people with severe and critical infections relies heavily on having access to affordable medicines that we can add to the steroids, oxygen and close supportive care that we already provide in our projects. As new treatments emerge, it will be simply inhumane if they remain unavailable in resource-limited settings, just because they are patented and too expensive.”
The WHO added what it said were “lifesaving” interleukin-6 receptor blockers to its list of treatments for COVID-19 last July. It recommended the use of corticosteroids in September 2020.
In recent weeks, government regulators have also approved new oral treatments for the disease, including Paxlovid, Pfizer’s antiviral pill, which showed close to 90 percent efficacy in preventing hospital admission and death in high-risk patients. It also retained its effectiveness with Omicron, the company said.
WHO recommends two new drugs to treat COVID-19 (14/01/2022) | @WHO
Omicron: What you need to know about the COVID variant (01/18/2022) | @CBSnews
Will current medicines still work?
Spokespeople for Merck and Pfizer both say they believe their antiviral pills, which have been hailed as potential game-changers in the fight against the virus, will likely remain effective against Omicron. Pfizer’s drug, Paxlovid, and Merck’s, molnupiravir, were both granted FDA emergency use authorization in December.
Of the monoclonal antibody treatments currently authorized for COVID-19, only two drugs — one made by AstraZeneca and one from GlaxoSmithKline and Vir Biotechnology — appear to remain effective against Omicron.
The Biden administration recently lifted curbs on the distribution of monoclonal antibodies from Regeneron and Eli Lilly, pointing to “significant variability” in Omicron’s prevalence around the country and new guidelines published by the National Institutes of Health on prioritizing limited supplies of the drugs.
Regeneron said on December 16 it was working on “next generation” monoclonal antibodies that could effectively treat Omicron cases, acknowledging that its current antibodies “have diminished potency against Omicron.”
2 charts show how Omicron symptoms differ from Delta and past coronavirus variants (01/14/2022) | @businessinsider
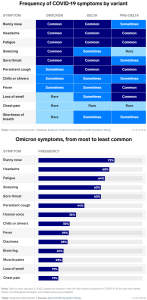
What are the symptoms of Omicron? (w YouTube; 18/01/2022) | ZOE
… The top 5 symptoms in both periods were: 1. runny nose; 2. headache; 3. fatigue (mild or severe); 4. sneezing; 5. sore throat
…the PREDICT program… people who ate a high-quality diet, rich in plant-based foods, were 10% less likely to get COVID-19 and 40% less likely to get severe COVID-19 requiring treatment in the hospital. …
Omicron Variant: What You Need to Know | @CDCgov
Coronavirus Self-Checker | @CDCgov
Coronavirus: 14 Omicron symptoms ranked from most to least prevalent (01/19/2022) | @timesofindia
Why ‘mild’ COVID symptoms aren’t what you think they are (01/18/2022) | @Deseret
Am I asymptomatic, or do I just really not want to have Covid-19? A guide. (01/18/2022) | @voxdotcom
Here’s One Early Omicron Symptom You Should Watch for as Infections Climb (w Video; 01/15/2022) | @nbcchicago
Omicron: What We Know About the New Coronavirus Variant (01/03/2022) | @nytimes
US faces wave of omicron deaths in coming weeks, models say (01/18/2022) | @AP
COVID-19 Scenario Modeling Hub
