All the below links and excerpts are in English.
Convalescent plasma(回復期血漿療法)に係るリンクと当方作成の抜粋を取り急ぎ以下のとおり貼っておきます。
The FDA has authorized convalescent plasma treatment for coronavirus patients – but some scientists worry it’s too soon (08/26/2020) | @businessinsider
During the 1918 Spanish flu pandemic, doctors discovered they could treat sick patients with the blood of those who had already recovered. …
Antibodies develop in plasma, the liquid portion of blood – they’re part of our body’s natural response to a foreign pathogen. So the idea behind the treatment is to help sick people mount an antibody response to the virus by transferring plasma intravenously from those who already have antibodies.
“What we really need are drugs that, when given early, can prevent a symptomatic person from requiring hospitalization or very dramatically diminish the time that they’re symptomatic,” …
… Plasma must be transferred quickly from a donor to a recipient – and both must have compatible blood types. The quantity is also limited, since it depends on blood donations. …
A national study of 35,000 hospitalized coronavirus patients, which is still awaiting peer review, found that patients less than 80 years old who weren’t on a respirator and received plasma containing high levels of antibodies within three days of their diagnosis had a 35% lower mortality rate than those who were treated four or more days after their diagnosis.
The process of creating hyperimmune globulin involves pooling plasma from recovered patients and heat-treating it so that any remaining pathogens get destroyed. The result is a vial of medicine with consistent antibody levels that can easily be administered to patients. The drug focuses on the most common antibody found in blood ? immunoglobulin G (IgG) – which usually confers long-term immunity.
… The Mount Sinai Hospital … working with Emergent BioSolutions, a Maryland-based biopharmaceutical company, to develop a hyperimmune globulin product. …
… “The Fight Is In Us” … The coalition hopes to secure regulatory approval from the FDA by the end of 2020. …
Why we don’t know if convalescent plasma works to treat Covid-19 (08/29/2020) | @qz
… “There’s no money to be made in plasma,” says Jeffrey Henderson, a physician and infectious disease researcher at Washington University St. Louis. Because it’s a biological product, it can’t be patented or sold for a profit. As a result, no single research group or company has funded a large, randomized controlled trial of plasma, the highest standard of clinical evidence. …
It isn’t cheap: Between 2012 and 2018, the median cost of bringing a drug to market was $985 million, about $19 million of which goes to clinical research. Typically, drug companies are happy to invest. Once their product is approved, they can sell it at a price that makes up for the loss (and then some). …
…expanded access program (EAP), a network spearheaded by researchers at the Mayo Clinic in Rochester, Minnesota…
… Eventually, the EAP program got some funding from the US Biomedical Advanced Research and Development Authority…
…Albert Einstein College of Medicine in New York…
Takeda, CSL-led alliance starts scaling up production of COVID-19 plasma therapy as phase 3 kicks off: report (10/13/2020) | @fiercepharma
… The clinical batches were produced at Takeda’s U.S. facility in Georgia and CSL’s site in Bern, Switzerland. The National Institute of Allergy and Infectious Diseases in the U.S. is running the trial. …
Eli Lilly and Regeneron have reported encouraging results for their antibody cocktails, each of which combines two synthetic monoclonal antibodies. While those products have less antibody variety, they only used donated plasma to help identify the antibodies with the most promise in fighting COVID. …
DOD Awards $750,000 to Plasma Technologies, LLC for Manufacturing of Convalescent Plasma Products Using a Novel Process in Support of the U.S. COVID-19 Response (08/17/2020) | DOD
… The Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND) partnered with HHS and the Army Contracting Command – Aberdeen Proving Ground (ACC-APG), to select Plasma Technologies, LLC for this cooperative agreement. Plasma Technologies, LLC, a plasma biologics technology company, is located in Charleston, South Carolina. …
Plasma Therapy Global Market Report 2020-30: Covid 19 Growth and Change (07/30/2020) | CISION
Major players in the plasma therapy market are Takeda Pharmaceutical Company Limited, Bio Products Laboratory Ltd., Arthrex, Inc., Biotest AG, China Biologic Products Holdings, Inc., DePuy Synthes Companies, CSL Limited, Grifols, S.A., Octapharma, and Terumo BCT, Inc. …
The global plasma therapy market is expected to grow from $187.67 million in 2019 to $246.95 million in 2020 at a compound annual growth rate (CAGR) of 31.6%. The growth is mainly attributed to the COVID-19 outbreak and the urgent need to treat a growing number of cases. …
… In March 2020, Takeda Pharmaceutical Company Limited has initiated a plasma-therapy, Anti-SARS-CoV-2 polyclonal hyperimmune globulin (H-IG), which is termed as TAK-888 for treating COVID-19. The TAK-888 utilizes the plasma collected from convalescent donors who have been cured of COVID-19 and is administered to the patient suffering from COVID-19.
CoVIg-19 Plasma Alliance Builds Strong Momentum Through Expanded Membership and Clinical Trial Collaboration (05/07/2020) | @TakedaPharma
… In addition to those announced at its inception – Biotest, BPL, CSL Behring, LFB, Octapharma and Takeda – the Alliance welcomes new industry members ADMA Biologics, BioPharma Plasma, GC Pharma, and Sanquin. …
In parallel, the Alliance has confirmed it will work with the National Institute of Allergy and Infectious Diseases (NIAID) at the NIH to test the safety, tolerability and efficacy of the hyperimmune therapy in adult patients with COVID-19. This global study is currently anticipated to start in the summer…
“Hyperimmune globulin therapy has the potential to be one of the earliest treatment options for COVID-19, and we look forward to working with NIAID and health authorities to bring this therapy to patients as early as possible,” …
… To amplify awareness, the Alliance has gained support from large organizations outside of the plasma industry. Examples of those offering resources to the Alliance include Microsoft and Uber Health. Microsoft is providing technology support, including the Alliance website and the Plasmabot for donor recruitment. The Plasmabot streamlines the process for a potential donor to quickly gain information about their nearest collection center from across the member network. In parallel, Uber Health has agreed to donate 25,000 round-trip rides to transport potentially eligible donors to and from plasma collection centers. …
Global Plasma Leaders Collaborate to Accelerate Development of Potential COVID-19 Hyperimmune Therapy (04/06/2020) | @TakedaPharma
Biotest, BPL, LFB, and Octapharma have joined an alliance formed by CSL Behring (ASX:CSL/USOTC:CSLLY) and Takeda Pharmaceutical Company Limited (TSE:4502/NYSE:TAK) to develop a potential plasma-derived therapy for treating COVID-19. The alliance will begin immediately with the investigational development of one, unbranded anti-SARS-CoV-2 polyclonal hyperimmune immunoglobulin medicine…
Trump admin funds plasma company based in owner’s condo (02/11/2020) | @ABCNews
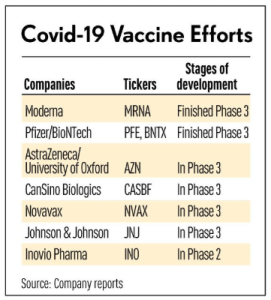
Plasma Technologies LLC, Pepcid, ApiJect Systems America, Novavax
ThermoGenesis and ImmuneCyte Joint Venture Developing Several Convalescent Plasma and Antibody Therapeutic Approaches Against COVID-19, Featured on FOX40 and Other Local News Outlets (04/24/2020) | @biospace
Takeda, Other Firms Test Covid-19 Convalescent-Plasma Treatment: NIH funds study of high antibody concentrations combined with the antiviral remdesivir (10/08/2020) | @WSJ