All the below links, excerpts, and tweets are in English.
昨日に引き続き取り急ぎ以下貼っておきます。
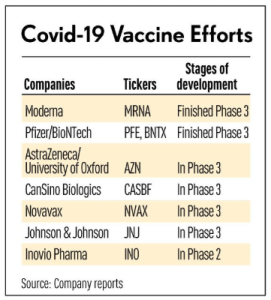
Multidose COVID-19 vaccines will test state tracking systems: People have to get two shots of the same type of vaccine (12/04/2020) | @verge
… Both take two doses, given a few weeks apart, and both could be circulating at the same time. And that’s just the two front-runners. Other multidose vaccines are still in the development pipeline.
There are a few ways states, doctors, and patients could keep the dosages straight, says Claire Hannan, executive director of the Association of Immunization Managers. In the simplest solution, everyone who gets a COVID-19 vaccine will also get a card that tells them which vaccine they had and when their next dose should be. But that’s the last line of defense in a complex vaccine tracking system. “As the backup to the backup to the backup, they’ll hand out cards,” Hannan says.
Ideally, the cards won’t be necessary because patients and doctors will be able to rely on electronic records and digital registry systems to keep track of who got which vaccine and when they got it. Through the pandemic, states have struggled with digital systems for other key areas, like COVID-19 testing – sometimes relying on fax machines and incomplete, handwritten forms to send information from testing sites to local health departments. But Hannan says the US immunization data collection systems at the state level are prepared to manage the expected crush of information from an unprecedented mass vaccination campaign like the one the country is about to start. Most tools were in place even before the pandemic hit. …
Big health systems with robust electronic health record management will likely have an easier time with this process than smaller clinics, says Howard Forman, a professor of public health, management, and economics at Yale University. “They’re used to keeping track of whether patients have had their followup appointments…
… Each state has its own Immunization Information System (IIS), a centralized registry that keeps track of every vaccine each person vaccinated in that state has received. Vaccination clinic software and electronic health records systems feed into those registries, which help doctors keep track of the vaccination records for individual patients, and also give each state big-picture data on vaccination rates in individual communities.
A state-level IIS can also notify doctors and patients when people are due for a second dose of a vaccine. They already use them to keep track of doses for the meningitis B vaccine…
The systems in place to track vaccinations today are much more advanced than they were in 2009, when the country struggled to distribute and track the H1N1 flu vaccine (which was only one shot), and electronic health records were rare. Only some states used their IIS programs. Many states had to rely on SurveyMonkey to connect with health care providers about vaccine delivery…
Moderna reaffirms 20M vaccine dose goal for December (12/04/2020) | @BosBizJournal
… The biotech has enlisted Italian firm Lonza to ramp up production to 500 million to one billion doses of the vaccine annually and Catalent Inc. to make all of the labels, seals and glass vials. Local life sciences unicorn Ginkgo Bioworks has also been helping Moderna optimize its production of raw materials for the drug.
Moderna has additionally hired another 150 employees to produce the vaccine out of its Norwood manufacturing facility. …
Moderna says its vaccine candidate has ‘potential’ to confer longer-term immunity (12/03/2020) | @MarketWatch
…National Institute of Allergy and Infectious Diseases…
The company said it remained confident it will have 20 million doses of the vaccine candidate available in the U.S. by the end of the year, and it expects to have between 100 million and 125 million doses available globally in the first quarter of next year.
Of these, between 85 million and 100 million will be available in the U.S. and between 15 million and 25 million will be available elsewhere…
Moderna’s 3-month data raise hopes for COVID-19 vaccine durability (12/04/2020) | @FierceBiotech
… Moderna shared details of the NEJM letter alongside an update on its effort to scale up production. …
Moderna Provides Updates on the Clinical Development and Production of Its COVID-19 Vaccine Candidate (12/03/2020) | @moderna_tx
… “mRNA-1273 produced high levels of binding and neutralizing antibodies that declined slightly over time, as expected, but they remained elevated in all participants three months after the booster vaccination.”…
Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination (12/03/2020) | @NEJM
What it feels like to get Moderna’s COVID-19 vaccine: “It’s not pleasant, but it’s definitely worth the risk of developing these side effects to make sure we can find an end to this pandemic.” (12/03/2020) | @BostonDotCom
Saginaw doctor who took part in Moderna vaccine trial seeks to reassure public (12/04/2020) | @MLive
Moderna Says COVID Vaccine Could Create Immunity For At Least Three Months (w Video; 12/04/2020) | @wbz
New data shows people who got Moderna vaccine still had antibodies 3 months later: The data came from a small number of phase 1 participants. (04/12/2020) | @ABCNews
Continuing COVID-19 vaccine trials may put some volunteers at unnecessary risk. Is that ethical? (w Video; 12/04/2020) | @USATODAY
No legal grounds for employers to force employees to get vaccinated, say experts (12/04/2020) | @CTVNews
Canada doubles Moderna vaccine order, daily COVID-19 cases could top 10,000 by January (12/04/2020) | @reuters
… Anand also said FedEx Corp and Innomar Strategies, a Canada-based division of AmerisourceBergen, had been contracted by the federal government to provide logistical support on vaccine delivery.
Moderna COVID vaccine best for Nunavut because of storage, shipping: top doctor (12/04/2020) | @CKOMNews
… Moderna’s vaccine is preferred because the Pfizer one requires cold storage and shipping would be too difficult in Nunavut. …
Two vaccines might get emergency approval this month. Here’s what you need to know (12/01/2020) | @latimes
What about those second doses?
The CDC’s vaccine tracking system will monitor who gets a first dose and will coordinate with Pfizer and Moderna on the shipment of the second doses. This means medical centers can administer all the doses they initially receive without having to hold onto half for the second shot.
How long before I can drive to my local pharmacy and get a vaccine?
If you’re healthy and a nonessential worker, you will have to wait. Most experts feel that the immunization effort in the United States will be in high gear by late spring and early summer of 2021…
Moderna to submit Covid-19 vaccine to FDA as full results show 94% efficacy (11/30/2020) | @statnews
… Moderna said that safety data is being reviewed continuously, but that there are no new serious safety concerns. The most common adverse events included site pain, fatigue, muscle or bone pain, headaches, and redness at the injection site. The reactions were more serious in the vaccine group after patients received a second dose. The results Pfizer and BioNTech released 12 days ago for their vaccine are roughly similar. …
… Each strand of synthetic mRNA is designed to encode for a protein found on the surface of SARS-CoV-2, the virus that causes Covid-19. Those mRNA strands enter the body’s cells and instruct them to produce that protein. The immune system then recognizes it as a foreign invader and produces antibodies that protect against Covid-19 if a person is later exposed to the virus.
The Covid-19 vaccine would be Moderna’s first product approved by the FDA and, assuming Pfizer’s vaccine is approved first, only the second mRNA medicine ever licensed. …
Moderna’s COVID-19 Vaccine Candidate Gets More Good News (w Voice; 11/30/2020) | @NPR
… Both use the same novel technology. Instead of injecting a weakened or dead virus, which is a common strategy for vaccines, these products are essentially small pieces of genetic material. When that’s injected into a person’s arm, it’s picked up by cells in the immune system. The cells read the genetic code and use that to produce a protein that is actually a key fragment of the coronavirus. The body then builds antibodies that latch onto that fragment, so if and when someone encounters the actual coronavirus, the body is primed to fight it off with antibodies. …
Could the FDA have moved even faster to review Covid-19 vaccines? @NicholasFlorko and I take a look.$PFE $BNTX $MRNAhttps://t.co/DTvlS3tcMd
— Matthew Herper (@matthewherper) December 4, 2020
We are expanding our particle engineering & drug product capabilities in our Bend (OR) site to support our partners' early-phase programs. Our investment will bring 11 new suites to improve program lead times. #SmallMolecules https://t.co/xIGdCMLeL4 pic.twitter.com/oDwtO2csY5
— Lonza Pharma & Biotech (@LonzaPB) December 3, 2020
#COVID19 is requiring a significant reduction in development & clinical trial timelines. Catalent Biologics’ Mike Riley addresses manufacturing challenges & discusses how collaborative partnerships are crucial. https://t.co/UE8ND6fssL
— Catalent Pharma (@CatalentPharma) December 3, 2020
COVID-19 is the greatest biological crisis of our generation. We are honored to be able to contribute our technology to efforts combating the pandemic and grateful for the support from the @DFCgov to expand our biosecurity platform. https://t.co/83sqXw5jtr
— Ginkgo (@Ginkgo) November 25, 2020
Our Chairman, President and CEO, Steve Collis, spoke with @thehill’s Steve Clemons about leveraging the #pharma #supplychain’s expertise during the ongoing #COVID pandemic. Watch here: https://t.co/nftGq06oaf
— AmerisourceBergen (@Healthcare_ABC) December 4, 2020
The executive director of the Association of Immunization Managers said states have not been given a date to expect shipments of coronavirus vaccines, but they’re preparing to receive them as early as December 11. https://t.co/bTkrFLNc95
— KEYT NewsChannel 3 (@KEYTNC3) December 1, 2020
The COVID-19 vaccine should go ‘into arms’ not ‘into storage’: Association of Immunization Managers Director https://t.co/t9tCBPfVQx via @Yahoo
— Raymond Gibbs (@raygibbs1) December 2, 2020
Dr. Mena Khan, assistant director of medical simulation at the Covenant HealthCare Simulation Center at @CMU_Medicine in Saginaw, reflected on her experience participating in phase three of the Moderna #COVID19Vaccine trial. Read more: https://t.co/fBc6H8kkcq @CMUniversity
— CMU Health (@CMU_Health) December 4, 2020