All the below links and excerpts are in English.
Tocilizumab/Atlizumab(Actemra®)に係るリンクと当方作成の抜粋を取り急ぎ以下のとおり貼っておきます。
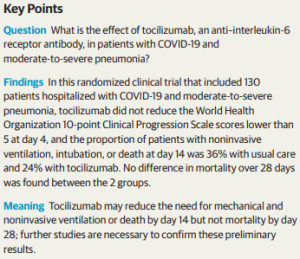
Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial (w PDF; 10/20/2020) | @JAMANetwork
PDF
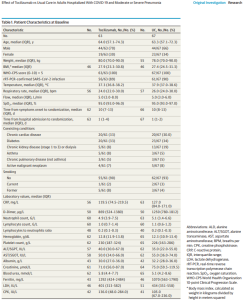
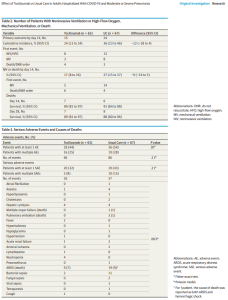
Efficacy and safety of tocilizumab in COVID-19 patients: A living systematic review and meta-analysis (w PDF; 11/05/2020) | @CMIJournal
Conclusion
Cumulative moderate certainty evidence shows that tocilizumab reduces the risk of mechanical ventilation in hospitalized COVID-19 patients. While RCTs showed that tocilizumab did not reduce short-term mortality, low certainty evidence from cohort studies suggests an association between tocilizumab and lower mortality. We did not observe a higher risk of infections or adverse events with tocilizumab use. This review will continuously evaluate the role of tocilizumab in COVID-19 treatment.
Efficacy of Tocilizumab on Patients With COVID-19 (08/24/2020) | @MGHMedicine,@genentech ClinicalTrials.gov @nlm_news
Brief Summary:
This is a randomized, double blind, multi-center study to evaluate the effects of tocilizumab compared to placebo on patient outcomes in participants with confirmed SARS-CoV-2 infection and evidence of systemic inflammation.
The aim of this study is to test the effect of Tocilizumab on multi-organ dysfunction in a phase 3 randomized controlled trial among hospitalized patients with COVID-19 infection.
Specifically, as compared to placebo, we will test whether tocilizumab is associated with a reduction in multi-organ dysfunction among hospitalized COVID-19 adult patients with elevated inflammatory measures. Multi-organ dysfunction will be measured as the incidence of the following composite endpoint (mechanical ventilation, renal replacement therapy, mechanical support, need for inotropes or vasopressors, liver dysfunction (increased bilirubin), and all-cause mortality). We will also assess multiple pre-specified secondary (exploratory) endpoints and safety endpoints.
We hypothesize that, as compared to placebo, tocilizumab will reduce transfer to the ICU, need for mechanical ventilation, increase rates of hospital discharge in patients diagnosed with severe COVID-19 infection and evidence of exaggerated inflammatory response.
New results from a once-promising therapy show the difficulty of treating covid-19: In one study, the drug, tocilizumab, reduced risk of death in ICU patients, but two others found negligible results with slightly different patients (10/21/2020) | @washingtonpost
Studies offer little hope for tocilizumab in treating COVID (10/20/2020) | @CIDRAP
Time to Reassess Tocilizumab’s Role in COVID-19 Pneumonia (10/20/2020) | @JAMANetwork
Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19 (10/20/2020) | @JAMANetwork
Efficacy of Tocilizumab in Patients Hospitalized with Covid-19 (10/21/2020) | @NEJM
Tocilizumab for COVID-19? Three Studies Yield Mixed Findings (10/20/2020) | @JWatch
Tocilizumab for patients with COVID-19 pneumonia. The single-arm TOCIVID-19 prospective trial (w PDF; 21/10/2020) | Journal of Translational Medicine
Appropriate use of tocilizumab in COVID-19 infection (w PDF; 10/2020) | International Journal of Infectious Diseases @ElsevierConnect
PDF
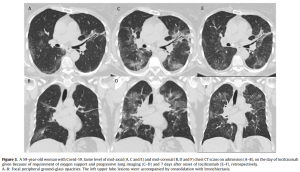
Roche’s Actemra helps keep coronavirus patients off ventilators despite earlier trial flop (09/18/2020) | @FiercePharma
…Roche is also pairing Actemra with Gilead Sciences’ antiviral Veklury, better known as remdesivir, in the phase 3 Remdacta trial. Eli Lilly’s JAK arthritis med Olumiant combined with Veklury helped patients recover faster in a recent trial run by the National Institute of Allergy and Infectious Diseases.
Tocilizumab/IL-6 Inhibitors | @RealTimeCOVID19
Case report: use of lenzilumab and tocilizumab for the treatment of coronavirus disease 2019. | @WHO
Decreased Mortality in Coronavirus Disease 2019 Patients Treated With Tocilizumab: A Rapid Systematic Review and Meta-analysis of Observational Studies (w PDF; 23/09/2020) | @OUPAcademic
Tocilizumab for treatment patients with COVID-19: Recommended medication for novel disease (09/16/2020) | @NCBI
Efficacy of tocilizumab in COVID‐19: A systematic review and meta‐analysis (w PDF; 12/09/2020) | @WileyGlobal
Glucocorticoids, tocilizumab may reduce complications in COVID-19-related cytokine storm (09/03/2020) | @HealioRheum
Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study (w PDF; 08/14/2020) | @TheLancetRheum
Tocilizumab in patients with severe COVID-19: a retrospective cohort study (w PDF; 08/01/2020) | @TheLancetRheum
Chugai Starts Phase III Clinical Trial of Actemra for COVID-19 Pneumonia in Japan (04/08/2020) | @chugai_cc
What is the role of the IL-6 inhibitor tocilizumab (Actemra) in the treatment of coronavirus disease 2019 (COVID-19)? (11/10/2020) | @Medscape