All the below links and tweets are in English.
Northern Europe Vol.5 (incl coronavirus, fish, 2019)
Denmark Vol.3 (pharmaceutical corporations: Genmab)
Denmark Vol.2 (pharmaceutical corporations: Novo Nordisk)
各国名や首都名 と Japan をツイッター検索に入れて出て来たもの を取り急ぎ以下貼っておきます。
https://twitter.com/UsherKomugisha/status/1256200466842689536
Gallery: Helsinki’s hanami festival – a slice of Japan in Finlandhttps://t.co/ZO9INlSm42#Finland #Japan #hanami #spring #cherryblossoms #culture #tradition
— Yle News (@ylenews) May 19, 2019
This year marks the centenary of Finish – Japanese diplomatic ties Discover the Nordic & East Asian interaction between artists in the 20th century in the exhibit Silent Beauty. Until October 6 at @AteneumMuseum in #Helsinki #Japan #Finland #Art pic.twitter.com/hANGTXl4tw
— ICEE ICOM (@ICEE_ICOM) August 21, 2019
Carved in snow and ice: Helsinki Cathedral rises in Japan https://t.co/cZTVBrJGb5 via @News Now Finland
— Nordic News (@Nordic_News) February 5, 2019
WINTER FOLKLORE IN COSTUMES Part 3 lovingly photographed by Charles Fréger (Austria, Japan, Slovenia, Finland ) No red Santa to be found! #GothicAdvent pic.twitter.com/cbgLfRHmXh
— Coffin Boffin (@DrSamGeorge1) December 12, 2020
Japan’s forest cover is 68.5%, placing it second behind Finland among OECD member countries.https://t.co/F8IjOyYzMb
— Nippon.com (@nippon_en) December 10, 2020
We are cancelling our flights to Tokyo from 26 March due to travel restrictions in Japan. We'll fly to Narita airport from Helsinki on Wed 25 March and back to Helsinki on 26 March. We intend to start flying to Haneda airport from 3 May, travel restrictions permitting.
— Finnair (@Finnair) March 23, 2020
We are adding Sapporo in Japan and Punta Cana in the Dominican Republic to our network for winter 2019/2020, with direct flights from Helsinki from mid-December until the end of March. https://t.co/CY9HmsjBSt pic.twitter.com/8qzQXNDk6l
— Finnair (@Finnair) January 14, 2019
Warm congratulations to Rica Maesawa, our first Signature Chef from #Japan. Rica’s restaurant Nanakusa in #Tokyo has gained #Michelin star https://t.co/qDwysbMDeE You can enjoy Rica’s meals onboard our flights from Japan to Helsinki @FinnairJapan pic.twitter.com/U0wcoyXd4c
— Finnair (@Finnair) December 11, 2018
New blog post: Flying Finnair Business Class A330-300 from Nagoya, Japan to Helsinki, Finland LINK: https://t.co/LtjqTriJ5Y #feelFinnair @Finnair #AVGeek pic.twitter.com/JZ12dzDHLb
— TRAVELDAVEUK (@traveldaveuk) October 9, 2018
Good news from Helsinki Airport! New direct flight connection between Helsinki and Sapporo, Japan, was opened last week. The route, operated by Finnair, is the only direct scheduled flight between Europe and Sapporo. Read more: https://t.co/g3mV3Rs4IW #FromHEL pic.twitter.com/CdTo8WsJ3v
— Helsinki Airport (@HelsinkiAirport) January 10, 2020
For five years, Japan Airlines has been operating daily between Helsinki Airport and Tokyo. We listed the most interesting and surprising facts about Japan Airlines: https://t.co/42YItElT20 pic.twitter.com/0kojd1Zcyt
— Helsinki Airport (@HelsinkiAirport) February 11, 2019
Finland and Japan are moving yet again a little bit closer as @Finnair starts flying to #Sapporo in December! Good news for fellow Europeans as well Helsinki being a handy hub for many. @Finnair @FinnairJapan @HelsinkiAirport @VisitHelsinki @OurFinland https://t.co/fqKkiL4iNM pic.twitter.com/aaiQFyBM3I
— Ari Honkanen (@arihonkane) January 14, 2019
https://twitter.com/arihonkane/status/1139719196059660288
https://twitter.com/sulake/status/1197897103458099200
Shinichi Nikkuni from Japan moved to #Helsinki to establish a fund that helps new Nordic companies grow with venture capital.
He chose #Finland because of its work-life balance and collaborative spirit inside the startup ecosystem.#StartupNationhttps://t.co/CbY9ggKNg4 pic.twitter.com/gBlPa3vT1v
— thisisFINLAND (@thisisFINLAND) November 29, 2020
https://twitter.com/thisisFINLAND/status/1127454625265856512
Silent beauty. It’s a phrase that perfectly captures the shared aesthetic of two remote parts of the world. One is the Nordic region, the other is East Asia. https://t.co/iuem6SENPm via @wallpapermag @AteneumMuseum #Finland #exhibition #Japan #Nordics #EastAsia #Helsinki #museum
— GoodNewsfromFinland (@goodnewsfinland) July 28, 2019
“While Japan decided to save all its banks, and many corporations, Finland was forced to accept a more difficult, yet ultimately more successful method of ‘cleansing’ its economy.” Economist Tuomas Malinen @mtmalinen at GnS Economics, Helsinki https://t.co/dC4XRNzPnt
— Greg Gaylor (@SMUMustangAlum) January 12, 2020
https://twitter.com/StanceFinland/status/1104077100284346368
#Iran FM @JZarif just arrived in Helsinki, Finland, for talks on EU's JCPOA commitments and regional issues. He tells reporters he's going to visit Norway and Sweden after Finland, and then travel to several Asian states (incl. Japan) for important coordination ahead of #UNGA pic.twitter.com/zKmvp4I9Tq
— Reza Khaasteh (@Khaaasteh) August 18, 2019
https://twitter.com/NIHTokyo/status/1321391430259085312
Hommage a Vincent van Gogh. This cherry tree park is located between an industrial area and a very ordinary Eastern Helsinki suburb. Local residents, activists for the park,were recently received by PM Abe of Japan in Tokyo. #Helsinki pic.twitter.com/CihaSTemeM
— Katri Viinikka (@ViinikkaK) May 12, 2019
https://twitter.com/RSBNetwork/status/1145679006693515264
https://twitter.com/TheVinum/status/1233749628304154625
Star power: Despite not being at his best, #YuzuruHanyu blew away the field at the Helsinki #GrandPrix.
"In the end it felt like a battle," said #Japan's 2-time #Olympic champ.
Story: https://t.co/1ZzOcit7SJ#hanyu #羽生結弦 #GPHelsinki #figureskating #フィギュアスケート pic.twitter.com/45rOpvjGbw
— Kyodo News Sports (@kyodo_sports_en) November 5, 2018
Japan's two-time #Olympic champion #YuzuruHanyu scores emphatic victory on #GrandPrix season debut in Helsinki.#hanyu #羽生結弦 #GPHelsinki #figureskating #フィギュアスケートhttps://t.co/1ZzOcit7SJ
— Kyodo News Sports (@kyodo_sports_en) November 4, 2018
Japan's two-time #Olympic #figureskating champion #YuzuruHanyu says he is in good shape ahead of his #GrandPrix season debut in #Helsinki#Hanyu #羽生結弦 #GPFigurehttps://t.co/op31G5Urlc
— Kyodo News | Japan (@kyodo_english) November 2, 2018
Congratulations to Stockholm Junior Water Prize winners Hiroki Matsuhashi & Takuma Miyaki from Japan. Check out their amazing project and video here https://t.co/t55gCk2xp1 pic.twitter.com/xKHEO8sW1x
— Xylem Inc. (@XylemInc) August 25, 2020
Japan Team Lands 2020 Stockholm Junior Water Prize https://t.co/8BobUDJ4zZ
— OOSKAnews water news (@OOSKAnews) September 1, 2020
https://twitter.com/olympipod/status/1301930209436938245
Sweden and difference with Nordics – and Japan too: https://t.co/XyRZhdEd7I
— Ivor Cummins (@FatEmperor) December 13, 2020
https://twitter.com/NIAS_1968/status/1331570328112394243
More on Lewis conveniently under-estimating SARS-CoV-2's infection fatality, or IFR, for:
– the Diamond Princess ship
– Great Britain (via Ferguson et al.)
– Stockholm, Sweden
– Tokyo, Japan
– China (via Verity et al.)https://t.co/zIQFVqid41 pic.twitter.com/h89FxkVIHi— Atomsk's Sanakan (@AtomsksSanakan) November 15, 2020
https://twitter.com/RacenewsService/status/1308827128495042561
A4: So many! The villages of Val d'Anniviers, #Switzerland. Himachal Pradesh, India. Niseko, Japan! Åre, Sweden. Why? For the skiing! (We're so predictable.) Superb skiing & marvelous small towns go hand in glove. We love them both. Pictured is Åre. #weekendwanderlust pic.twitter.com/y7IexuYcv4
— Ski Travel Go (@skitravelgo) December 4, 2020
Canada, Norway, Sweden, the United States and Japan.https://t.co/ZZhOyiz2Nt
— Lady J of Tay (@brawday) December 4, 2020
Just made a video of our every day life here in Stockholm. Apart of Sweden, Japan, Iceland and South Korea for ex. never had a quarantine either.. and the numbers here per million are much lower than in many other countries.. time to go back to life and stop the madness! pic.twitter.com/cJTua4iDYS
— Bruno Simões (@simoesbrn) May 21, 2020
https://twitter.com/ViratUpadhyay99/status/1337858049864654848
A #Japan #Sweden #Canada combo; that would make for an interesting equity portfolio @chigrl https://t.co/o4pMNdw6DR
— Chris Metcalfe (@chris_metcalfe1) November 15, 2020
Next time someone illustrates Japan or even Tokyo with an image of Shibuya I'm going to respond with this reality check.
I got lots of wistful nostalgia seeing this in my feed but can't explain it. It's like aesthetic Stockholm syndrome https://t.co/FTgu7ODLfz
— 無理です (@_infotherapy) July 6, 2020
Queen Silvia and Princess Sofia of Sweden were joined by Queen Sofia of Spain and Princess Takamado of Japan for the 3rd Dementia Forum X at the Royal Palace of Stockholm #OnThisDay last year: https://t.co/BdGo0LK9UK
— The Royal Watcher (@saadsalman719) May 15, 2020
They are back in stock – GRAMICCI Tees – Buy them online at https://t.co/3uu7ZLO7Rr
•••
@theterracestockholm @gramicci @gramicci_jp #theterracestockholm #gramicci #japan @ The Terrace Stockholm https://t.co/Xf5Ph4ALoj— The Terrace Stockholm (@theterracesthlm) December 2, 2020
Pop density of Stockholm higher than many UK cities, Japan didn't implement blanket lockdown either
It's about targeted, sustainable long-term measures. Virus here, need to live with it – we can't lockdown for yearshttps://t.co/YJyAgBmJEF— help (@scotlandwakeup) September 30, 2020
#Study finds just 7% of #people in #Stockholm had #Covid antibodies https://t.co/Z754wjTcdr @MailOnline #bigdata #healthCare #WHO#dataScientist #dataScience #Linux #FRENCHtech #100daysofCode #RStats #Javascript #France#USA☯️ #canada #london #Australia #japan #FemTech #UK
— Chidambara .ML. (@chidambara09) May 23, 2020
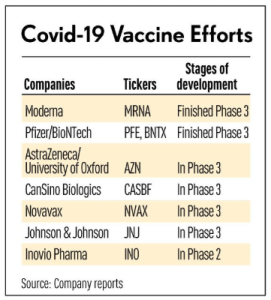
Congrats to our partners @Genmab presenting at the Japan Society of Clinical Oncology #JSCO. Whether in Kyoto or not, their virtual booth is accessible for all to explore. #cancelcancer https://t.co/ggN3lUC06l
— 3FX (@3FXinc) October 23, 2020
We are pleased to be at the Japan Society of Clinical Oncology. Join us for a symposium on #cervicalcancer on Oct. 22 and visit our virtual booth to learn more about our mission to transform #cancer treatment. https://t.co/PxO0RXDpRh #JSCO20 pic.twitter.com/WbytiOndgZ
— Genmab (@Genmab) October 22, 2020
#Daratumumab now also available for #transplant eligible patients in #Europe, another frontline approval.
The press release also reveals another important frontline approval in #Japan, for the #MAIA regimen (Dara-Len-Dex). @Genmab $GMAB $JNJ Multiple #Myeloma https://t.co/tTlRIuUM8a
— E Lambrechts (@EgidiusLambrech) January 21, 2020
@JanssenGlobal @Genmab #Darzalex (daratumumab + VMP) #DVMP approved in #Japan for Frontline #MultipleMyeloma #NDMM@IMFmyeloma @theMMRF @MyelomaMultiple @multiplemyeloma https://t.co/LikDdOLwcA
— TheOncoForum (@TheOncoForum) August 22, 2019
Genmab Announces Submission of Supplemental New Drug Application for Daratumumab in Front Line Multiple Myeloma in Japan https://t.co/sYfEQSlgmB
— Genmab (@Genmab) December 14, 2018
#PRESS Product approved in Japan for the treatment of type 2 diabetes
— Novo Nordisk (@novonordisk) June 29, 2020
#NEWS: We file for regulatory approval of once-weekly semaglutide for the treatment of type 2 diabetes in Japan https://t.co/9De7LH2zJy pic.twitter.com/JDT9E5OGQw
— Novo Nordisk (@novonordisk) February 28, 2017
Congrats @Health2Sync on partnering with @novonordisk in Japan!! Read on to see how their shared mission could improve outcomes for people with diabetes: https://t.co/IcY9QDgQW7 pic.twitter.com/uK8sWsUwAM
— TaiwanStartupStadium (@startup_stadium) April 21, 2019
#RA #Pharma #Update@novonordisk has partnered with @Health2Sync , a Taiwan-based company, to help promote the latter’s #digital #diabetes management app, #SyncHealth app, in #Japan
To know more, click: https://t.co/LzhzrMEUMt— Roots Analysis (@RootsAnalysis) March 15, 2019
#Japan has more 10 million estimated #diabetes patients in the country, and @Health2Sync is looking to help this population through the new partnership with @novonordisk.https://t.co/lHIfBWn0T8
— Cherubic Ventures (@cherubicvc) March 6, 2019
And here is PIONEER 10, Oral Semaglutide H2H vs 0.75 mg Dulaglutide in Japan-HbA1c below 6.5% achieved by 21%, 43% and 58% of PPL on treatment with 3, 7 and 14 mg oral semaglutide, respectively, compared to 41% of the people treated with 0.75 mg dulaglutide over 57 wks #T2D pic.twitter.com/h4saysiHxx
— Daniel J Drucker (@DanielJDrucker) September 20, 2018
Physicist Nishina Yoshio (仁科 芳雄) was born #OTD in 1890. He spent time at Cambridge and Copenhagen in the 1920s, where he contributed to the development of quantum electrodynamics, then returned to Japan and established a national program of modern physics research. pic.twitter.com/KBqgruJOB4
— Robert McNees (@mcnees) December 6, 2020
I have been the chairman of Japan-Denmark Parliamentary Friendship League. I succeeded DPM ASO Taro. https://t.co/zeQcMJltFp
— KONO Taro (@konotaromp) November 14, 2020
https://twitter.com/ImperialJPNfan/status/1333236809321508864
The Ambassador of Australia, Canada, Denmark, European Union Delegation, France, Germany, Italy, Japan, Netherlands, Norway, Sweden, Switzerland, Turkey, United Kingdom, and United States of America say: STOP GENDER-BASED VIOLENCE!#GenerationEquality #16DaysofActivism #EndGBV pic.twitter.com/2TNruZWIJ2
— Winnie Estrup Petersen (@DKAmbBD) December 3, 2020
https://twitter.com/bopinion/status/1318652262110105612
Mark Meadows says 'we are not going to control the pandemic". Why the F not? Japan, S.Korea, Germany, New Zealand, Taiwan, China, Canada, Poland, Portugal, Ireland, Denmark, etc all control COVID so why can't America Mark? The answer is Trump! https://t.co/EfUQ86lsen
— In Joe we trust! (@JohnLukeSam1) October 25, 2020
.@IOA_UWA hosted the 17th Mike Carroll Travelling Fellowship presentation last night! Guests heard from 2018 & 2019 recipients – #UWA PhD students Yueqi Zhang (who travelled to the Czech Republic), Suyog Subedi (Denmark) & @SoodehTirnaz (Japan). @uwanews @UWAresearch @KadambotS pic.twitter.com/smkrOnQEIC
— UWA Institute of Agriculture (@IOA_UWA) November 13, 2020
Deep & interesting conversation with @ambshimokawa #Japan is a valued partner of #NATO – demonstrated yesterday when Japan along with other close partners participated in the NATO ministerial. #Denmark is proud to serve as NATOs contact point for Japan from Jan next year. pic.twitter.com/HRCRnOFr7X
— Liselotte Plesner (@LPlesner) December 3, 2020
https://twitter.com/EFlygenring/status/1324219943722774532
The Watanabe Fund aim is to strengthen the academic ties between Iceland and Japan. It supports @uni_iceland students & staff to study and work in Japan, and Japanese students & researchers can apply for grant for a stay in Iceland. Deadline 15 January.https://t.co/01neAOrWyY pic.twitter.com/3uddEGM0Om
— University of Iceland (@uni_iceland) November 24, 2020
"The sovereign is still at A, its sixth highest rating, and on par with Japan and Iceland, according to a statement on Monday."https://t.co/4AeQ12Jsn2
— Vivian Nereim (@viviannereim) November 10, 2020
Falling in lava with volcanoes as a kid ignited a life-long passion that has led to a PhD for this #MasseyGrad! Her passion has taken her to Iceland, Japan & NZ. Where will @charlinator_as go next? https://t.co/8VlJtPHgfs pic.twitter.com/p5N2J9Jln6
— Massey University (@MasseyUni) October 27, 2020
https://twitter.com/CultureTrip/status/1256244490920177670
Japan and Norway are live from fish – as producers and consumers. Very good meeting with Japan Fisheries Association, on common interests and future cooperation. (And my face mask is not decorated with jumping salmon, these are Japanese goldfish!)@NorwayinJapan @Seafood_Norway pic.twitter.com/mXTem8KCxg
— Inga M. W. Nyhamar (@NorAmbTokyo) July 2, 2020
Canada, France, Northern Ireland, Sweden, Denmark, Germany, Portugal, England, Japan, the Republic of Ireland, Wales, Finland, Norway, Scotland and the United States are celebrating the International Year of the Salmon. https://t.co/lOgnKte1H1
Photo Credit USFWS Edward Steenstra pic.twitter.com/HzpwazwvnY
— USFWS Fisheries (@USFWSFisheries) May 1, 2019
Norway had industrial-sized freezers full of salmon. Japan loved sushi. The Japanese just needed to learn to love raw salmon. https://t.co/k2VOJNK14C
— NPR's Planet Money (@planetmoney) June 7, 2019
Eating raw salmon wasn’t a thing in Japan for a long time. Norway, of all places, figured out a way to change that. https://t.co/QCkiWMIjGL
— NPR's Planet Money (@planetmoney) June 6, 2019
Best #sales story ever? – how Norway convinced Japan to love salmon sushi. http://t.co/Cou3o4KGL7 pic.twitter.com/XZynQBEpzJ
— Techspace® (@techspace) September 21, 2015
Well… the flavour profile isn’t that different from various Japanese fermented foods so there’s potential with the right marketing. Just like Norway convinced Japan to eat Salmon sushi: https://t.co/gc67QdnQx2
— Janek Mann (@janekm) August 11, 2020
https://twitter.com/GunvarLWie/status/1302869565664374785
Live king crab and fresh salmon travel direct from Norway's Arctic waters to consumer plates in Korea, China and Japan – and with growth in the #seafood industry, DHL Global Forwarding has doubled capacity on our Oslo to Seoul route. #DHL https://t.co/A744eOi0VL pic.twitter.com/HOSohAIqT0
— DPDHL News (@DeutschePostDHL) August 17, 2018
Next up @paFroyen, director of @InnovasjonNorge Japan, with a talk on #facilitating #innovation between #Norway and the world through the #Nordic #Innovation #House initiative https://t.co/9I3EdROaBB . Follow their Tokyo actives at @NIHTokyo. @forskningsradet pic.twitter.com/8IvUrIxsnz
— Alexander Rothkopf (@rothkopfAK) June 22, 2020
https://twitter.com/bbcworldservice/status/1333037912779272193
https://twitter.com/Seasaver/status/1247618468297543684
https://twitter.com/juliana_monty/status/1321576500810948609
https://twitter.com/LicypriyaK/status/1261917723325390848
https://twitter.com/HSIUKorg/status/1247819772475183104
https://twitter.com/sethpiper/status/1144892089949462528
https://twitter.com/animal_leaks/status/1305144582724898819
https://twitter.com/RacingXtinction/status/1248464975930126336
https://twitter.com/StarboyHK/status/1336528890160791552
https://twitter.com/OECDEduSkills/status/1333500043291021312
https://twitter.com/GlobPeaceIndex/status/1335188429789913089
Just 19 countries have net-zero targets in law: Austria, Bhutan, Costa Rica, Denmark, Fiji, Finland, France, Hungary, Iceland, Japan, Marshall Islands, New Zealand, Norway, Portugal, Singapore, Slovenia, Sweden, Switzerland and the UK
— Assaad Razzouk (@AssaadRazzouk) November 20, 2020
How much does a cinema ticket cost in your city?#movies #Zurich #Switzerland #London #UK #NY #NewYork #US #Copenhagen #Denmark #Tokyo #Japan #Helsinki #Finland #sanfrancisco #Oslo #Norway #riyadh #KSA #SaudiArabia #sydney #australia #cinema #movie #film pic.twitter.com/ioQeDfI6Ft
— Al Bawaba Business (@AlBawabaBiz) September 24, 2019